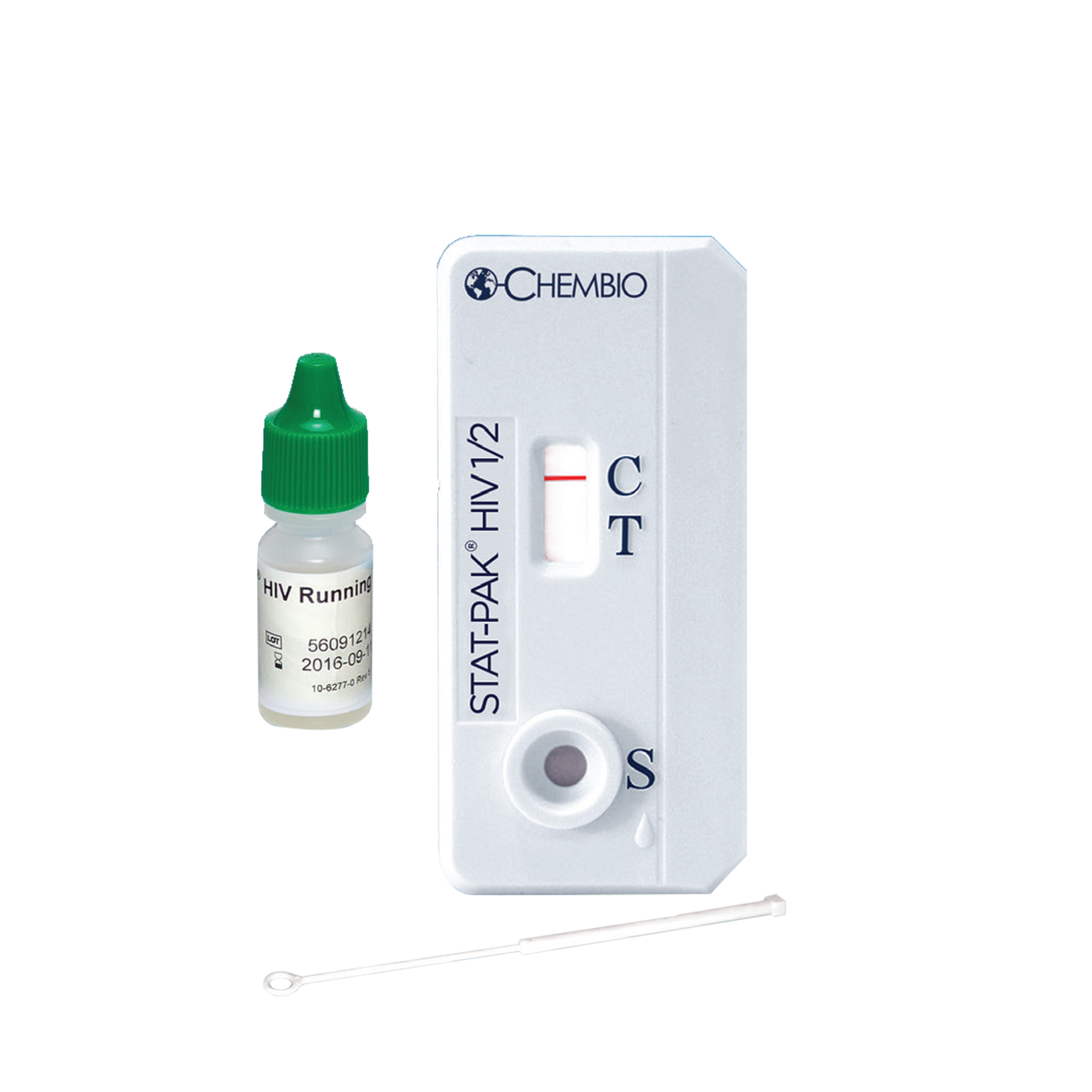
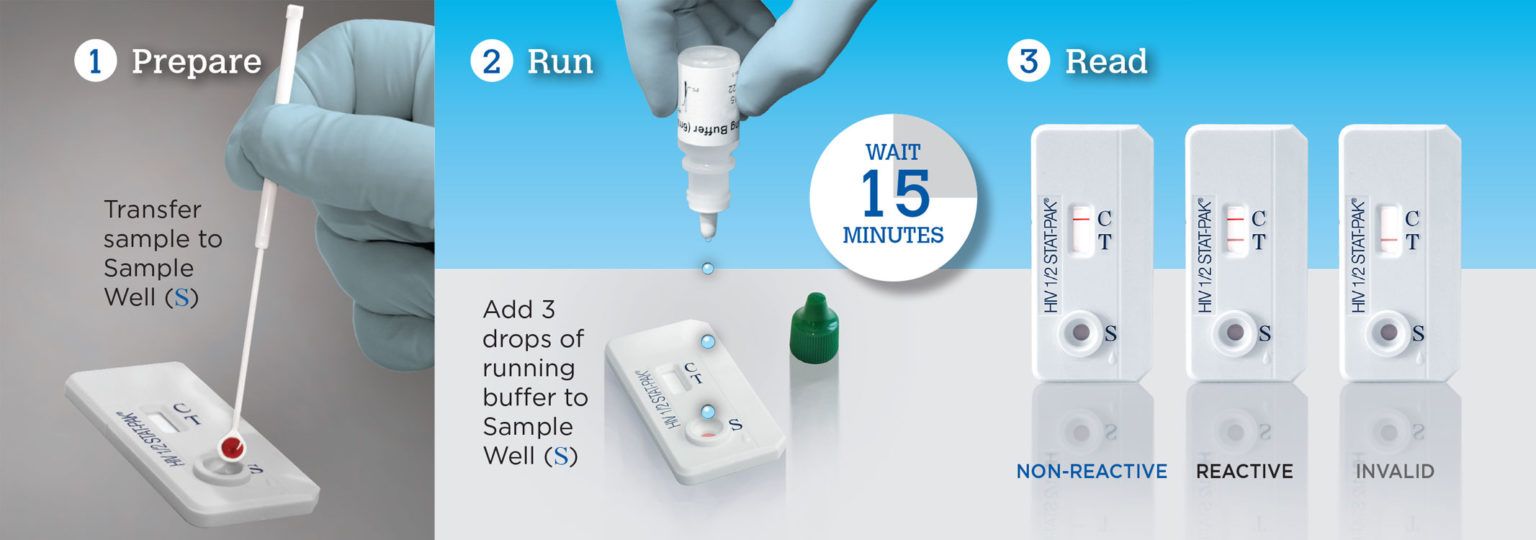
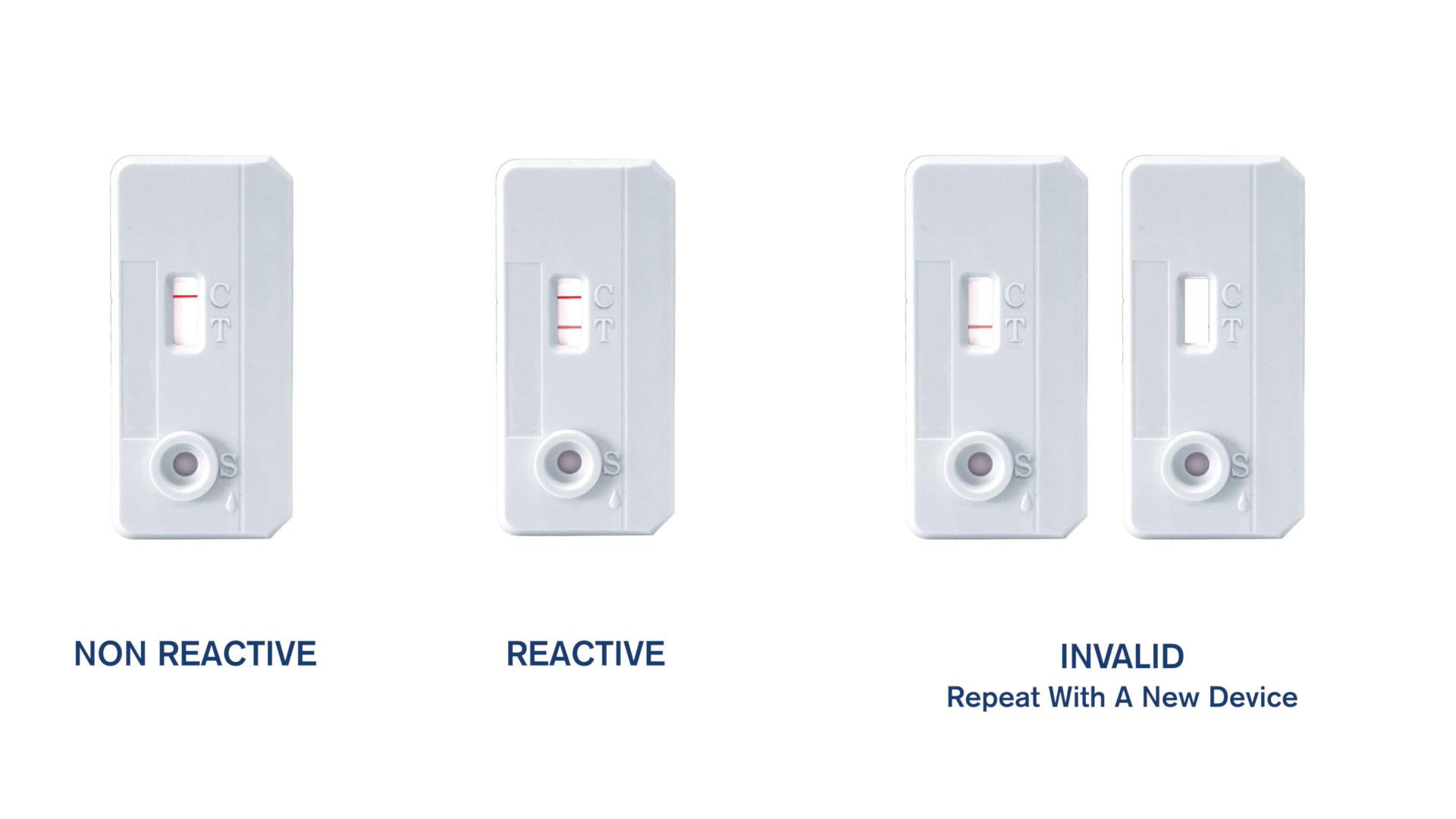
STAT-PAK® HIV 1/2 Assay: Rapid HIV Test in 3 Easy Steps
CHEMBIO HIV 1/2 STAT-PAK® Assay

WHO Prequalified

FDA Approved

CE-Marked
- Small sample volume:5μl
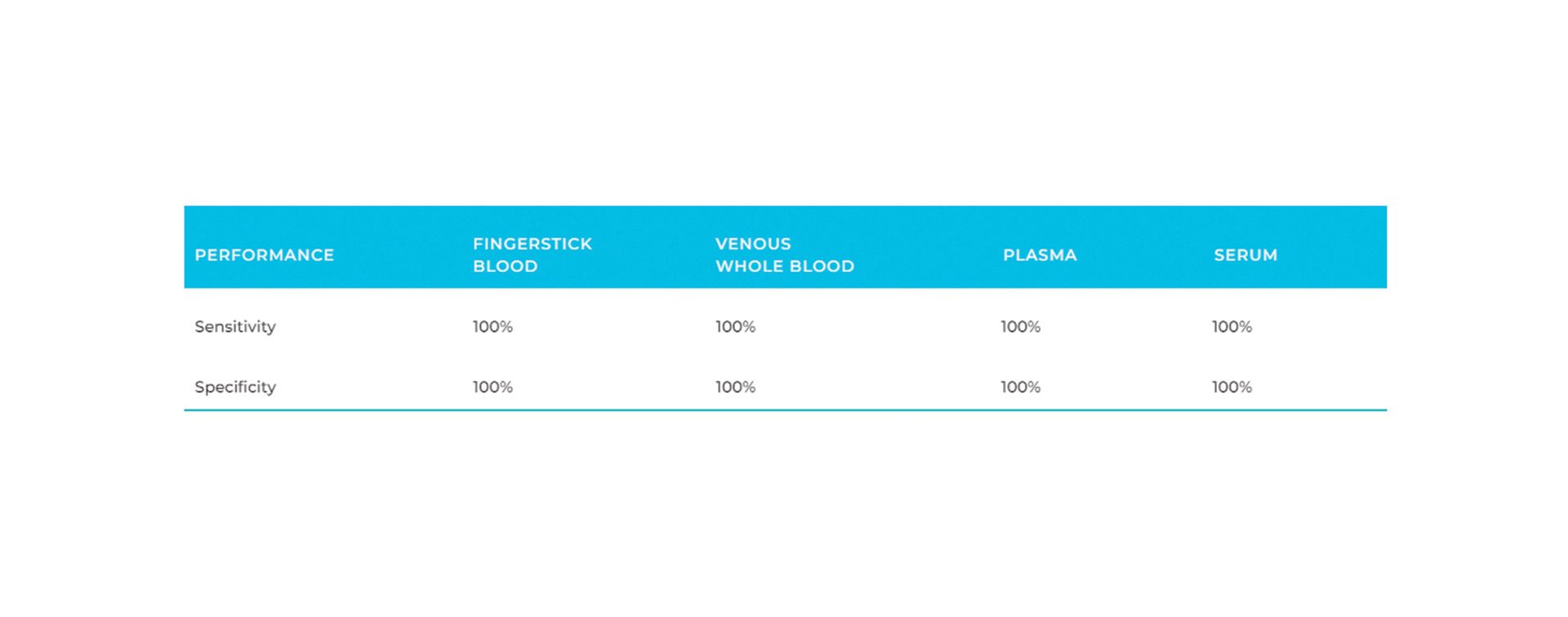
- Sensitivity: 100%; Specificity: 100%²
- Built-in procedural control
1 Kosack CS et al. Journal of the International AIDS Society 2017, 20:21345
2 Refer to product insert for details
A rapid point-of-care assay for the detection of HIV-1 and HIV-2 antibodies in fingerstick whole blood, venous whole blood, serum or plasma.
| Platform | Immunochromatographic Assay |
| Format | Cassette |
| Detection | HIV-1 and HIV-2 antibodies |
| Specimen | Whole blood, serum & plasma |
| Sensitivity | 100% |
| Specificity | 100% |
| Assay Time | 15 minutes |
| Shelf Life* | 24 months |
*From date of manufacture
Do you still have questions?
For Technical Support please call
1300 418 188 9am-7pm AEST / 9am-8pm AEDT, 7 days per week
Or contact us via the form below and a member of the team will get back to you.
To contact your local state/territory health department click on the following link:
https://www.health.gov.au/about-us/contact-us/local-state-and-territory-health-departments